Along with NCERT Solutions For Class 9 Science Chapter 1 Matter in Our Surroundings, we also provide Notes and Lesson Plan made specially by our Study Rankers for better understanding. Students may read the NCERT Science Chapter 1 PDF to practice concepts with NCERT Solutions and Extra Questions and Answers by keeping in mind the latest CBSE curriculum. Shine among your friends after scoring high in Quiz, MCQ, and Worksheet.
Table of Contents
Notes For Class 9 Science Matter in Our Surroundings
(Also find NCERT Solutions For Class 9 Science Matter in Our Surroundings given below)
Introduction
- Anything which occupies space and has mass is called matter. Air and water, sugar and sand, hydrogen and oxygen, etc.
- Ancient Indian philosophers said that all matter, living or non-living was made up of five basic elements (panch tattva): air, earth, fire, sky, and water.
- Modern-day scientists classify matter in two ways: on the basis of its physical properties and on the basis of its chemical properties.
- On the basis of physical properties, the matter is classified as solids, liquids, and gases.
- On the basis of chemical properties, the matter is classified as elements, compounds, and mixtures.
Physical Nature Of Matter
- Matter is made up of particles.
- Everything around us is made up of tiny pieces or particles. The particles of matter are constantly moving (they are in motion).
- The particles which make up matter are atoms and molecules.
Evidences of Particles in Matter
The evidence for the existence of particles in matter and their motion comes from the experiments on diffusion and Brownian motion.
Also Read : Wind Summary Class 9 English
Characteristics of Particles of Matter
The important characteristics of particles of matter are the following:
- The particles of matter are very, very small.
- The particles of matter have space between them.
- They are constantly moving.
- The particles of matter attract each other.
The Particles Of Matter Are Very, Very Small:
- The very, very small size of particles of matter can be shown by performing the following experiment by using potassium permanganate and water.
- Take 2-3 crystals of potassium permanganate and dissolve them in 100 ml of water in a beaker. We will get a deep purple colored solution of potassium permanganate in water.
- Take out approximately 10 ml of this solution and put it into 90 ml of clear water in the second beaker.
- Due to this dilution, the color of the potassium permanganate solution in the second beaker becomes a bit lighter.
- Take out 10 ml of this solution and put it into another 90 ml of clear water in the third beaker. The color of the solution will become still lighter.
- Keep diluting the solution like this 5 to 8 times.
- In this way, we get a very dilute solution of potassium permanganate in water but the water is still colored.
- This experiment shows that just a few crystals of potassium permanganate can color a large volume of water.
- So we conclude that there must be millions of tiny particles in just one crystal of potassium permanganate, which keeps on dividing themselves into smaller and smaller particles.
The Particles Of Matter Have Space Between Them:
- The spaces between the particles of matter can be shown by performing the following experiment by using water and sugar.
- Take a 100 ml beaker.
- Fill half the beaker with water and mark the level of water.
- Dissolve some sugar (50gm) with the help of a glass rod.
- We will find that the level of sugar solution in the beaker is at the same mark where the water level was initially in the beaker.
- When sugar is dissolved in water, its crystals separate into very fine particles. These particles of sugar go into the spaces between the various particles of water due to which there is no change in the volume of water on dissolving sugar in it.
- The fact that there is no change in volume on dissolving sugar in the water tells us that there are spaces between the particles of water.
The Particles Of Matter Are Constantly Moving:
- The best evidence that particles of matter are constantly moving comes from the experiments on diffusion and Brownian motion.
The Particles Of Matter Attract Each Other:
- There are some forces of attraction between the particles of matter which bind them together.
- The force of attraction between the particles of the same substance is cohesion.
- If we take a piece of chalk, a cube of ice, and an iron nail, and beat them with a hammer, we will find that it is very easy to break the piece of chalk into smaller particles, it requires more force to break a cube of ice, whereas the iron nail does not break at all even with a large force.
- This shows that the force of attraction between the particles of chalk is quite weak; the force of attraction between the particles of ice is a bit stronger whereas the force of attraction between the particles of the iron nail is very, very strong.
States Of Matter
On the basis of physical state, all the matter can be classified into three groups: Solids, Liquids and Gases.
Properties of Solids, Liquids and Gases
| S.No. | Solids | Liquids | Gases |
|---|---|---|---|
| 1. | Solids have a fixed shape and fixed volume | Liquids have fixed volume but they have no fixed shape | Gases have neither a fixed shape nor a fixed volume |
| 2. | Solids cannot be compressed much | Liquids cannot be compressed much | Gases can be compressed easily |
| 3. | Solids have high densities | Liquids have moderate to high densities | Gases have very low densities |
| 4. | Solids do not fill their container completely | Liquids do not fill their container completely | Gases fill their container completely |
| 5. | Solids do not flow | Liquids generally flow easily | Gases flow easily |
| 6. | For example: Ice, coal, wood, stone, iron, etc. | Water, milk, fruit juice, ink, petrol, etc. | Air, oxygen, hydrogen, nitrogen, steam, etc. |
Diffusion
- The spreading out and mixing of a substance with another substance due to the motion of its particles is called diffusion.
- The diffusion of one substance into another substance goes on until a uniform mixture is formed. For example diffusion of bromine vapors in air.
- Diffusion is the property of matter which is based on the motion of its particles. It occurs in gases, liquids and solids.
- Diffusion is fastest in gases and slowest in solids.
- The rate of diffusion increases by increasing the temperature of the diffusing substance.
Diffusion in Gases:
- Diffusion in gases is very fast. This is because the particles in gases move very quickly in all directions.
- The rate of diffusion of a gas, however, depends on its density. Light gases diffuse faster than heavy gases.
- Example: When we light an incense stick (agarbatti) in a corner of our room, its fragrance spreads in the whole room very quickly due to the diffusion of its smoke into the air.
Diffusion in Liquids:
- Diffusion in liquids is slower than that in gases. This is because the particles in liquids move slowly as compared to the particles in gases.
- Example: The spreading of the purple color of potassium permanganate into the water, on its own, is due to the diffusion of potassium permanganate particles into water.
- Note: Gases like carbon dioxide and oxygen are essential for the survival of aquatic plants and animals. The carbon dioxide and oxygen gas present in the air diffuse into water and dissolve in it. The aquatic plants use the dissolved carbon dioxide for preparing food by photosynthesis and aquatic animals use the dissolved oxygen of water for breathing. This is an example of the diffusion of gases into a liquid.
Diffusion in Solids:
- Diffusion can also take place in solids is a very slow process.
- Example: If we write something on a blackboard and leave it uncleaned for a considerable period of time, we will find that it becomes quite difficult to clean the blackboard afterward. This is due to the fact that some of the particles of chalk have diffused into the surface of the blackboard
Change of State of Matter
- We can change the physical state of matter in two ways:
- By changing the temperature (heating or cooling).
- By changing the pressure (increasing or decreasing the pressure).
- Solid to Liquid Change: Melting
- Liquid to Gas Change: Evaporation
- Gas to Liquid Change: Condensation
- Liquid to solid change: Freezing
Latent Heat
- The heat energy which has to be supplied to change the state of a substance is called its latent heat.
- Latent heat does not raise (or increase) the temperature. But latent heat has always to be supplied to change the state of a substance. The word ‘latent’ means ‘hidden’.
- The latent heat which we supply is used up in overcoming the forces of attraction between the particles of substance during the change of state. Latent heat does not increase the kinetic energy of the particles of the substance, so the temperature of a substance does not rise during the change of state.
- It is of two types:
- Latent heat of fusion
- Latent heat of vaporization.
- L.H. of Fusion (solid to liquid change):
- The heat which is going into ice but not increasing its temperature is the energy required to change the state of ice from solid to liquid (water). This is known as the latent heat of fusion of ice (or latent heat of melting of ice).
- Latent Heat of Vaporization (liquid to gas change):
- The latent heat of vaporization of a liquid is the quantity of heat in joules required to convert 1 kilogram of the liquid (at its boiling point) to vapor or gas, without any change in temperature.
Evaporation
- The process of a liquid changing into vapour (or gas) even its boiling point is called evaporation.
- The wet clothes dry due to evaporation of water present in them. Common salt is also recovered from sea-water by the process of evaporation.
Factors Affecting Evaporation:
The evaporation of a liquid depends mainly on the following factors:
- Temperature: The rate of evaporation increases by increasing the temperature of the liquid.
- The surface area of the liquid: The rate of evaporation increases by increasing the surface area of the liquid. For e.g. If the same liquid is kept in a test tube and in a china dish, then the liquid kept in the china dish evaporate more rapidly.
- The humidity of Air: The amount of water present in the air is represented by a term called humidity. When the humidity of air is slow, then the rate of evaporation is high, and water evaporates more readily.
- Wind Speed: The rate of evaporation of liquid increases with increasing wind speed.
Cooling caused by evaporation:
- The cooling caused by evaporation is based on the fact that when a liquid evaporates, it draws or takes the latent heat of vaporization from ‘anything’ which it touches. By losing heat, this ‘anything’ gets cooled.
- During hot summer days, water is usually kept in an earthen pot (called pitcher or Matka) to keep it cool. The earthen pot has a large number of extremely small pores (or holes) in its walls. Some of the water continuously keeps seeping through these pores to the outside of the pot. This water evaporates (changes into vapor) continuously and takes the latent heat required for vaporization from the earthen pot and the remaining water. In this way, the remaining water loses heat and gets cooled.
- Perspiration (or sweating) is our body’s method of maintaining a constant temperature.
- We should wear cotton clothes on hot summer days to keep us cool and comfortable.
NCERT Solutions Class 9 Science Matter in Our Surroundings
(Also find NCERT Solutions Class 9 Science Matter in Our Surroundings FAQs given below)
In Text Questions (Page 3)
Q. 1. Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, cold-drink, smell of perfume.
A) From the above list, matter are: Chair, Air, Almonds, and Cold-drink.
Q. 2. Give reasons for the following observation:
The smell of hot sizzling food reaches you several meters away, but to get the smell from cold food you have to go close.
A) The smell of hot sizzling food reaches several meters away, because the gaseous and aromatic articles of hot food have more kinetic energy and hence can travel freely and faster than the particles of cold food.
Q. 3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
A) The presence of intermolecular space in a liquid (here, water), is the reason why a diver is able to cut through water in a swimming pool.
Q. 4. What are the characteristics of the particles of matter?
A) The characteristics of the particles of matter are:
- Particles have intermolecular space.
- They have an intermolecular force.
- They of matter move continuously.
In Text Questions (Page 6)
Q. 1. The mass per unit volume of a substance is called density (density = mass/volume). Arrange the following in order of increasing density: air, exhaust from chimneys, honey, water, chalk, cotton and iron.
A) Increasing order of density is as follows:
Air < Exhaust from chimneys < Cotton < Water < Honey < Chalk < Iron.
Q. 2. (a) Tabulate the differences in the characteristics of states of matter. (b) Comment upon the following: rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
A) (a) Difference in the characteristics of 3 states of matter.
| Characteristics | Solid | Liquid | Gases |
| Shape & Volume | A solid has a fixed shape and volume. | A liquid has no fixed shape but has a fixed volume. | Gases neither have fixed shape nor a fixed volume. |
| Intermolecular Force | The particles in a solid have the maximum force of attraction between them. | The particles in a liquid, even though they have a strong force of attraction, it is quite less than found in solids but higher than that found in gases. | Force of attraction between particles present in gases is almost negligible. |
| Rigidity/ Fluidity | Particles in a solid cannot move freely; therefore, they are rigid and cannot flow. | Particles in a liquid can move freely and can therefore flow and are not rigid. | Particles found in a gas are in constant random motion moving in all directions and can, therefore, flow and are not rigid. |
| Compressibility | Solids are incompressible due to the tightly-packed molecules. | Liquids, even though they are almost incompressible, they can be compressed to a certain extent due to the little spaces present between the molecules. | Gases are highly compressible due to the presence of large spaces between the particles. |
| Density | Solids have the highest density. | Liquids have a lower density than solids, but more than gases. | Gases have very low density. |
| Kinetic Energy | The molecules present in solids possess the least amount of kinetic energy. | The molecules present in a liquid have kinetic energy higher than that in solids but lesser than gases. | The molecules in gases possess the highest amount of Kinetic energy. |
(b)
- Rigidity: It is the tendency of a substance to retain its shape on exposure to an outside force.
- Compressibility: The tendency of the particles to come closer to the application of external force is called compressibility. Gases and liquids are compressible.
- Fluidity: Fluidity is the property of particles to flow. Liquids and gases are fluid.
- Filling of a gas container: Gases have particles that move freely and randomly in all directions. Hence, a gas can fill the container.
- Shape: Solids have the maximum intermolecular force and have a rigid shape. But liquids and gases are fluid and takes the shape of the container.
- Kinetic energy: The energy possessed by particles due to their motion is called kinetic energy. As molecules of gases move freely and randomly, they have maximum kinetic energy.
- Density: The density of a substance is mass per unit volume, the solids have the highest density.
Q. 3. Give reasons:
- A gas fills completely the vessel in which it is kept.
- A gas exerts pressure on the walls of the container.
- A wooden table should be called a solid.
- We can easily move our hand in the air but to do the same through a solid block of wood we need a karate expert.
A)
- The molecules of gas have high kinetic energy and they are fluid and move freely in all directions. Due to these reasons, they fill the vessel completely in which they are kept.
- The molecules of the gas are in constant random motion due to high kinetic energy. These molecules constantly move freely and hit the walls of the container exerting pressure on its walls.
- The particles of the wooden table are solid and are tightly packed. They lack intermolecular space, it cannot be compressed, it is not fluid. So a wooden table is solid.
- We can easily move our hand in the air but to do the same through a solid block of wood we need a karate expert because gases have larger intermolecular spaces whereas solids have negligible intermolecular spaces. Hence, more force is required to break through a solid.
Q. 4. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
A) The molecules in ice make a cage-like structure with plenty of vacant spaces which allows it to float on water.
In Text Questions (Page 9)
Q. 1. Convert the following temperature to Celsius scale:
(a) 300 K (b) 573 K
A) (a) 300 – 273 = 27°C (b) 573 – 273 = 300°C
Q. 2. What is the physical state of water at:
(a) 250°C (b) 100°C
A) (a) At 250°C water is in the form of gas (b) At 100°C water is in the form of liquid as well as gas, as this is its boiling temperature.
Q. 3. For any substance, why does the temperature remain constant during the change of state?
A) During the change of state of any matter heat is supplied to the substance so as to overcome the force of attraction between the particles. At this point, the temperature remains constant. This extra heat is acquired by the molecules by latent heat to change from one state of matter to the other state.
Q. 4. Suggest a method to liquefy atmospheric gases?
A) A cylinder with a piston fitted on it will cool and apply pressure on gases leading to liquefication of gases.
In Text Questions (Page 10)
Q. 1. Why does a desert cooler cool better on a hot dry day?
A) The outer walls of the cooler are sprinkled with water constantly. This water evaporates due to hot dry weather. Evaporation causes cooling of inside air of cooler. A fan sends this cool air into the room.
Q. 2. How does the water keep in an earthen pot (matka) become cool during summer?
A) The earthen pot is porous with a lot of pores on it, the water oozes out through these pores and the water evaporates at the surface of the pot thereby causing a cooling effect. This makes the pot cold and the water inside the pot cools by this process.
Q. 3. Why does our palm feel cold when we put some acetone or petrol or perfume on it?
A) Acetone, petrol or perfume evaporate when they come into contact with air. The evaporation causes cooling sensation in our hands.
Q. 4. Why are we able to sip hot tea or milk faster from a saucer rather than a cup?
A) Tea in a saucer has a larger surface area than in a cup. The rate of evaporation is faster with the increased surface area. The cooling of tea in the saucer takes place sooner than in a cup. Hence we are able to sip hot tea or milk faster from a saucer rather than a cup.
Q. 5. What type of clothes should we wear in summer?
A) We should wear light-colored cotton clothes in summer. Light color because it reflects heat. Cotton clothes because it has pores in them, which absorb sweat and allows the sweat to evaporate faster thereby giving a cooling effect.
Textbook Exercises: NCERT Solutions Class 9 Matter In Our Surroundings
Q. 1. Convert the following temperatures to the Celsius scale.
(a) 293 K (b) 470 K.
A) (a) 293 – 273 = 20°C (b) 470 – 273 = 197°C
Q. 2. Convert the following temperatures to the Kelvin scale.
(a) 25°C (b) 373°C.
A) (a) 25 + 273 = 298 K (b) 373 + 273 = 646 K
Q. 3. Give a reason for the following observations.
- Naphthalene balls disappear with time without leaving any solid.
- We can get the smell of perfume sitting several metres away.
A)
- Naphthalene balls undergo sublimation and directly changes into vapour state without leaving behind any solid.
- We can get the smell of perfume sitting several metres away because perfume is gaseous and diffuse faster.
Q. 4. Arrange the following substances in increasing order of forces of attraction between the particles—water, sugar, oxygen.
A) Oxygen<Water<Sugar.
Q. 5. What is the physical state of water at—
(a) 25°C (b) 0°C (c) 100°C
A) (a) At 25°C, water is in a liquid state. (b) At 0°C, water is in a solid or liquid state as this is its freezing point. (c) At 100°C, water is in a liquid and gas state as this is its boiling point.
Q. 6. Give two reasons to justify
- Water at room temperature is a liquid.
- An iron almirah is a solid at room temperature.
A)
- Water at room temperature is a liquid because its freezing point is 0°C and boiling point is 100°C.
- An iron almirah is a solid at room temperature because the melting point of iron is higher than room temperature.
Q. 7. Why is ice at 273 K more effective in cooling than water at the same temperature?
A) Ice at 273 K will absorb latent heat from the medium to become water. Hence the cooling effect of ice is more than the water at the same temperature because water does not absorb this extra heat from the medium.
Q. 8. What produces more severe burns, boiling water or steam?
A) Steam at 100°C will produce more severe burns as extra latent heat is present in it.
Q. 9. Name A, B, C, D, E and F in the following diagram showing change in its state.
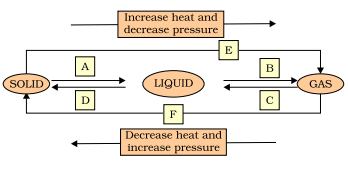
A) A – Melting
B – Evaporation
C – Condensation
D – Solidification
E – Sublimation
Some Frequently Asked Questions
(Here you can find NCERT Solutions Class 9 Science Matter in Our Surroundings FAQs.)
Q. 1. When 50 g of sugar is dissolved in 100 mL of water, there is no increase in volume. What characteristic of matter is illustrated by this observation?
A) Particles of water have intermolecular spaces between them into which sugar particles fit easily.
Q. 2. Name the phenomenon that occurs when Dettol is added to water.
A) Diffusion.
Q. 3. Why do we see water droplets on the outer surface of a glass containing ice cold water?
A) The water vapor present in the air comes in contact with the cold surface of the glass and condenses into droplets of water.
Conclusion: NCERT Solutions For Class 9 Science Chapter 1 Matter in Our Surroundings
Above written includes NCERT Solutions For Class 9 Science Chapter 1 Matter in Our Surroundings , detailed Explanation, and Question Answers. Browse our site for various detailed and easy NCERT Solutions and CBSE Notes.



