NCERT Class 7 Science Chapter 6 Physical and Chemical Changes, here we are going to give you a summary of this chapter along with notes which may be referred to during the examination time in order to revise the chapter in short time duration. In this chapter, we will study Physical and Chemical Changes along with examples and we will also know about a few terms or processes that are related to Physical and Chemical Changes.
Table of Contents
NCERT Class 7 Science Chapter 6 Physical and Chemical Changes: Introduction
We notice several changes happening all around us every day. Making curd from milk, dissolving sugar in water, rusting iron, boiling water, and melting ice, are just a few examples of changes that we see around us in day-to-day life. These changes can be categorized into two parts, namely: Physical Change and Chemical Change. Let us discuss these topics in detail for a better understanding.
Also refer to NCERT Class 7 Science Chapter 3 Fibre to Fabric Summary and Notes Pdf

NCERT Class 7 Science Chapter 6 Physical and Chemical Changes: Summary
- Changes are of two types,i.e, physical and chemical changes.
- Physical changes are changes that affect the physical properties of substances. No new substances are formed in these changes and are generally reversible in nature.
- Chemical changes are changes that tend to the formation of one or more new substances and are generally irreversible in nature.
- Iron can tend to form rust if exposed to oxygen and water.
- Rusting can be prevented by galvanization, which is a process by which iron or steel is covered by coating zinc to prevent water or oxygen or both from directly coming in contact with the metal.
- By crystallizing certain compounds from their solutions, they can be acquired in their pure form.
NCERT Class 7 Science Chapter 6 Physical and Chemical Changes: Notes
Physical Change
A change in which no new substance is formed and which can generally be reversed by reversing the conditions is called a physical change.
Here, only physical properties like shape, size, color and state of a substance is changed.
Eg: Melting of ice
Now let us discuss the term physical reaction which is important from an examination point of view–>
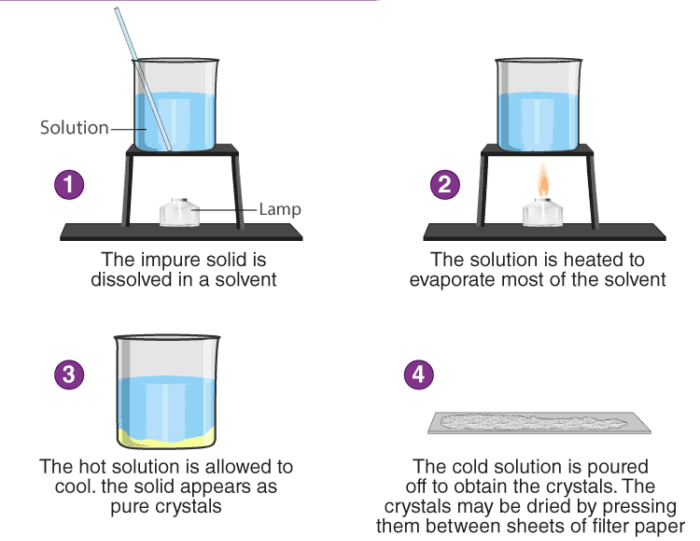
- Crystallization: Crystallization is the process of removing salts from their solution. It is a method of purification that isolates crystals from impure materials or cleans saltwater. It is considered under physical change.
Chemical Change
A change in which one or more new substances are formed and which cannot be reversed by reversing the conditions is called a chemical change. A chemical change is also called a chemical reaction.
Now let us discuss some terms under chemical reaction which are important from examination point of view–>
- Rusting: When something is said to be rusting, it means that it has formed rust on the surface of an iron object or structure. Rust is a compound formed from a reaction between iron and oxygen along with presence of water.
Rusting accelerates if the air has a high moisture content, or if it is more humid. Rusting weakens, flakes and degrades the strength, appearance, and permeability of iron.
This process can be represented by the following equation:
Iron (Fe) + Oxygen (O₂) (From air) + Water (H₂O) → Rust (Iron oxide, Fe₂O₃)

- Galvanization: Rusting can be prevented if the iron surface is blocked from contacting oxygen or water or both. A protective zinc coating is applied to iron or steel, to prevent rusting. This process is called galvanization.

Some other key terms
- Sublimation: Phenomenon in which solids directly covert into vapors on being heated. Eg: Camphor (Also known as Kapoor)
- Melting: Phenomenon in which solid converts into liquid on being heated. Eg: Ice
- Freezing: Phenomenon in which liquid converts into solid on being cool. Eg: Water
- Vaporization: Phenomenon in which liquid converts into gas on being heated. Eg: Water
- Condensation: Phenomenon in which gas converts into liquid on being cool. Eg: Water vapor
Also, refer to NCERT Class 7 Science Chapter 6 Physical and Chemical Changes detailed explanation
NCERT Class 7 Science Chapter 6 Physical and Chemical Changes PDF Download
NCERT Class 7 Science Chapter 6 Physical and Chemical Changes: Conclusion
In conclusion, we learned a lot about NCERT Class 7 Science Chapter 6 Physical and Chemical Changes. As a result, we can easily classify the day-to-day changes happening in our environment on our own. NCERT Class 7 Science Chapter 6 Physical and Chemical Changes notes have been made by our subject matter experts.
Frequently Asked Questions(FAQs) on NCERT Class 7 Science Chapter 6 Physical and Chemical Changes
The formation of metal oxides is an example of what kind of change?
State some observations while burning a magnesium ribbon.
List the chemical equation of burning magnesium ribbon.
List some observations that can happen during a chemical change.
(2) Sound may be produced.
(3) A change in smell may take place or a new smell may be given off.
(4) A color change may take place.
(5) A gas may be formed.