We shall learn in CBSE NCERT Class 7 Science Chapter 4 Heat that we can determine how hot something is by using our sense of touch. However, it may not always accurately indicate how hot something is. As a result, we will get knowledge of temperatures and thermometers. First, we’ll discover that a thermometer is a tool used to measure temperatures. Additionally, we shall examine several thermometer kinds. For example, learning about the maximum-minimum thermometer, laboratory thermometer, and clinical thermometer would be a few. As a result, we will understand when to use one and how to analyze one. Additionally, we will discover the safety measures to be followed when utilizing the same. After that, we’ll examine how to use the last two appropriately.
Through the heat chapter of the class 7 notes, we will understand the idea of heat transmission. We will be able to determine that heat moves from a hotter body to one colder. Additionally, there are three different methods for warmth to go from one thing to another. Conduction, convection, and radiation are a few of them. We will also examine heat conductors, both good and harmful. We will also discover why we dress in dark colors in the winter. We’ll also learn why we dress in bright colors in the summer. To fully understand distinct topics, we will also engage in various activities.
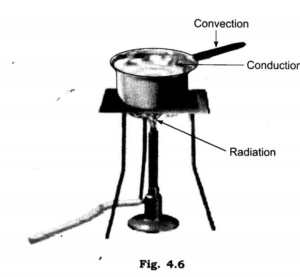
Table of Contents
NCERT Class 7 Science Chapter 4 Heat Introduction
Have you ever felt warm in the sun and freezing inside your house during the winter? Additionally, do you experience summertime heat inside and outside your home? We dress in wool-based clothing to keep ourselves protected from the bitter cold. These woolen garments come from animals that produce wool, such as sheep, goats, yacks, etc. We will feel calmer if we wear light-colored clothing in the heat. We will study this chapter to determine the object’s temperature and determine how hot it is. A temperature is a unit of measurement for the amount of heat a substance contains. It is measured using a tool called a thermometer.
NCERT Class 7 Science Chapter 4 Heat Summary
A form of energy transfer occurs when two objects, one hot and the other cold, come into touch. The name of this energy is heat energy. Joule is the SI unit for heat (J). The old measurement unit is the calorie. The temperature of an object refers to how hot or cold it is. Kelvin is the SI unit of temperature (K). The thermometer is the name of the device used to measure temperature.
Clinical Thermometer: The capillary, or narrow tube, of a clinical thermometer, is often constructed of thick glass.
- The capillary tube’s tip is a tiny mercury-filled glass bulb.
- Because mercury is liquid across a wide temperature range, it is ideal for thermometer use. In addition, given that it is a silvery gray color, it is relatively simple to see. But, unfortunately, it doesn’t adhere to the glass it contains.
- The scale covers several degrees on each side of the typical body temperature of approximately 37 degrees Celsius on a clinical thermometer.
- It is capable of measuring temperatures between 35 and 42 degrees Celsius.
Digital thermometer: People use digital thermometers to measure their body temperatures.
- They operate on little, dry cells.
- Because it is free of harmful mercury, the gadget is safer.
Scales of temperatures
The Celsius scale
was created by Anders Celsius sometime around 1743. He concluded that the freezing point of water is 0 degrees, and the boiling point is 100 degrees. Between these two locations, there are 100 divisions.
The Fahrenheit scale
was created by Daniel Gabriel Fahrenheit. Degrees Fahrenheit (F) are the scale’s units. Upon that scale, the freezing point for water is assumed to be 320 degrees Fahrenheit, while the boiling point is considered to be 2120 degrees Fahrenheit. Between these two locations, there are 180 divisions.
Kelvin scale
Lord Kelvin created this scale. The scale is expressed in K. Water’s freezing point is 273 K, whereas its boiling point is 373 K according to this scale.
Until both reach the same temperature, heat energy constantly moves from a higher temperature zone to a lower temperature region. This process is referred to as heat transfer. Conduction, convection, and radiation are the three basic methods of heat transport.
You may also like : Heat – Wikipedia
NCERT Class 7 Science Chapter 4 Heat Notes
Cold and Heat
- Using our sense of touch, we can determine if something is hot or cold. However, it occasionally misleads us. So, we make use of a thermometer.
- The temperature of a thing determines how warm or cold it is.
Thermometer:
This instrument measures how hot a thing is. In other terms, a thermometer is used to determine an object’s temperature.
Clinical thermometer
It is a tool used to measure an individual’s internal body temperature.
A glass tube with a consistent thickness makes up the object.
A mercury-filled bulb is located at one end of the glass tube.
Body temperature is shown by the mercury volume in the thermometer rising in the thread-like part.
Reading the scale on the thermometer will allow you to determine the Mercury level.
The thermometer’s scale measures temperature in degrees Celsius; the normal range for human body temperature is 35°C to 45°C.
A person’s body temperature is typically 37 °C.
The clinical thermometer has one slight, sharp curve (kink) near the bulb. This stops the mercury level in the thermometer from dropping on its own.
How should I use a clinical thermometer?
The thermometer should first be cleaned using an antiseptic solution.
The thermometer provides a few shocks before taking the temperature to lower the mercury level below 35o C.
After that, the thermometer is positioned for roughly a minute beneath the tongue.
Then you may remove it and check the thermometer’s temperature reading.
When utilizing a clinical thermometer, safety measures must be performed.
The thermometer must be disinfected after each usage.
Before taking the temperature, ensure the thermometer’s temperature is below 35 degrees Celsius.
To accurately determine the Mercury level, keep the thermometer level.
It must always be handled carefully to prevent breakdown. The thermometer’s bulb shouldn’t be touched at all.
Laboratory thermometer
Instead of measuring body temperature, the laboratory thermometer is often used to measure the temperature of other items like water. It is capable of measuring temperatures between -10°C and 110°C.
What safety measures need to be performed while using a lab thermometer?
Always take the same safety measures as when using a clinical thermometer.
The laboratory thermometer should always be held straight up, untitled, and in an upright posture.
Never let the thermometer’s bulb come in contact with the surface of the vessel containing the material.
But the thermometer’s bulb has to be fully submerged in the material surrounding it.
The Movement of Heat
Heat transfer typically occurs from a hot object to a cold thing.
Conduction
is heat transfer from a hot object to a cold entity. While certain materials can transfer heat, others are unable.
Conductors: The things that may let heat pass through them are called conductors. Metals like copper and aluminum, for instance.
Insulators: Insulators are things that block the passage of heat away from them. Wood and plastic, for instance.
Convection:
This term refers to the movement of heat in gasses and liquids. The first molecules of the liquid or gas to be heated are those closest to the heat source. The heat makes them lighter, and they rise. Next, the heavier, colder particles take their position, and so on until the entire liquid or gas becomes heated. Because of this, the region above a candle’s flame appears hot, but the area to its sides does not.
Radiation:
Heat is transferred by radiation, a process that involves waves. As an illustration, radiation from the sun heats the Earth’s surface. Each heated object releases some heat into the surrounding space. As a result, an object can frequently become heated simply by being close to a hot thing.
NCERT Class 7 Science Chapter 4 Heat Pfd Download
NCERT Class 7 Science Chapter 4 Heat Conclusion
People prefer to wear white or light-colored clothing on warm summer days because these colors reflect less heat from the sun and help us stay relaxed and comfortable. On chilly winter days, however, people recommend wearing dark clothing because these hues reflect more heat from the sun and help us stay warm.
We may thus conclude that dark things absorb heat more effectively and radiate heat more effectively than light ones. Let’s investigate this idea using the provided task as a base.
We dress in wool during the winter. Unfortunately, wool doesn’t transfer heat well. In addition, the air has been stowed away between the wool fibers. This air blocks heat transfer from our bodies to the chilly environment. As a result, we are heated.
Assuming the supplied, You may benefit by downloading the free PDF of Chapter 4 of the CBSE Class 7 Science Notes. Please leave a comment below with any questions you may have about NCERT Class 7 Science Notes Chapter 4 Heat, and we will respond as soon as possible.